Featured Faculty
Professor of Strategy; Herman Smith Research Professor in Hospital and Health Services Management; Director of Healthcare at Kellogg
When Merck, maker of Vioxx, voluntarily took the drug off the market in 2004, few realized that one consequence would be a reduction in the number of near-elderly men in the workforce.
Although a handful of studies have looked at the impact of anti-depressants on workforce participation, none had looked at similar effects in other classes of drugs until Craig Garthwaite, an assistant professor of management and strategy at the Kellogg School of Management, decided to use the withdrawal of Vioxx from the market as a natural experiment to address larger questions about the economic value of drugs that enhance quality of life.
Currently, the U.S. Food and Drug Administration does not always evaluate the potential economic impacts of a drug, but that might be a mistake; as more and more drugs on the market are designed to improve the quality of life rather than simply extend its length, Garthwaite argues, their impact on the ability of aging Americans to stay in the workforce is a salutary effect that could impact everything from how an individual weighs the risks of taking a drug to the way in which employers structure their health plans.
Vioxx is an anti-inflammatory drug of the class known as Cox-2 inhibitors, which includes its primary competitor in the marketplace, Celebrex. Cox-2 inhibitors help provide relief to individuals with diseases of chronic inflammation, such as osteoarthritis. They are uniquely powerful weapons against these conditions; in a 2006 survey of elderly arthritis sufferers, 18 percent of individuals reported that they would continue to take Vioxx if it were still available, even though they overestimated the elevated risks of a heart attack by nearly 50 percent.
In addition to their effectiveness at relieving the symptoms of painful inflammatory diseases, Cox-2 inhibitors offer a second advantage in comparison to older types of anti-inflammatory drugs: They do not increase the risk of gastro-intestinal (GI) bleeding. An estimated 16,500 people in the U.S. die every year from GI bleeding as a result of taking traditional nonselective anti-inflammatory drugs such as ibuprofen and naproxen.
A Lack of Options
When Vioxx was withdrawn from the market, studies show that many individuals who had been taking it did not switch to another anti-inflammatory drug. In addition to the possibility that it was over-prescribed, these studies suggest its primary competitor, Celebrex, was an imperfect Vioxx substitute for many patients. Garthwaite wondered what had been the impact of the sudden withdrawal of an apparently uniquely effective medication on a statistic with which economists are quite familiar: workforce participation, or the percentage of a population that is currently working.
To unravel the effects of Vioxx on Americans aged 55 to 75, Garthwaite used data from the Medical Expenditure Panel Survey (MEPS), a large-scale survey of individuals across time that records all of their prescriptions, as well as socio-economic data such as employment status. (MEPS is administered by the Agency for Healthcare Research and Quality, part of the U.S. Department of Health and Human Services.)
“Qualitatively, you see that Vioxx changed the number of people in the labor force.” — Craig Garthwaite
“Qualitatively, you see that Vioxx changed the number of people in the labor force [aged 55 to 75],” Garthwaite points out. That effect is even more pronounced for men—and larger still for men in physically demanding occupations when compared to that for men with non–physically demanding occupations. There was no statistically significant estimated effect on the likelihood of women to work. This could be a result of the kind of jobs held by women in this age group, which tend to be less physically demanding than those held by men (e.g., in construction). It could also be a sign that the medication does not work as well in women.
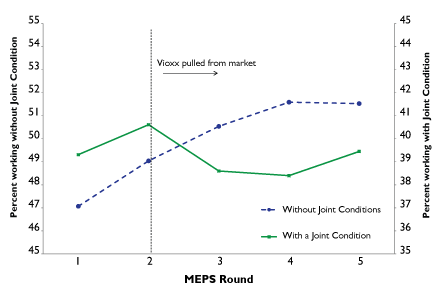
Figure 1. The percentage of people aged 55-75 in the workforce with and without joint conditions. MEPS rounds are the reporting periods for the survey. For reference, Vioxx was pulled from the market in September 2004.
In his study, Garthwaite was able to control for one obvious confounding factor, which was the possibility of a reverse causality in the relationship between Vioxx and work. In other words, it is possible that patients were working in order to get health insurance so they could afford life-enhancing drugs such as Vioxx, which Garthwaite estimates cost patients upwards of $1,000 a year, rather than finding themselves able to work because they were on Vioxx.
To eliminate this possibility, Garthwaite examined a number of other relatively expensive life-enhancing drugs that did not affect patients’ ability to work, such as the anti-cholesterol drug Lipitor and the erectile-dysfunction drug Viagra, and found that they did not correlate with employment.
Survey data about perceived quality of life are readily available for drugs like Vioxx, but Garthwaite says that workforce participation may be a better measure because it shows what is known as a “revealed preference.”
“[As economists,] when we see your actions, we see your underlying preference,” Garthwaite says. “So if someone is working, for them, the benefits outweigh the costs.” He thinks that having data about the economic effects of medical innovations might someday be relevant to how individuals evaluate the risk of taking a drug with known side effects.
The issue is a charged one, and it would require that patients have full information about the potential dangers of the medication. But at least one example of this informed tradeoff already exists in the market: The multiple sclerosis drug Tysabri, which was pulled off the market and then put back on, cannot be prescribed unless a patient has first registered with a special risk-management program.
Weighing the Risks
Although the economic impact of a drug is significantly further down the list of public concerns about pharmaceuticals than the effectiveness of the regulatory process in safeguarding health, Garthwaite says that work like his could be part of the larger debate over whether or not Vioxx should be available to patients.
“At some point, you want to come to grips with, in a cost benefit sense, [the question] ‘Should Vioxx be on the market?’ ” Garthwaite asks. “To the degree that Vioxx is the only effective pain medication for some individuals, there’s perhaps more of an argument that Vioxx should be on the market. Every drug has side effects, but on the flip side,16,500 people die from ibuprofen every year, which is also not a good outcome.”
Garthwaite is careful to point out that because his research addresses only one of the drug’s potential benefits, he does not take a stand in his paper on the issue of whether or not Vioxx once again should be made available to patients. Ultimately, he notes, the status of Vioxx is about a society’s—and its individuals’—tolerance for risk.
Related reading on Kellogg Insight
Fetal Influence on Familiar Ailments: The effect of in-utero conditions on long term health
The Effects of Health On Wealth: Major illness leads to financial catastrophe for the uninsured
Rational Rationing: Allocating scarce resources in uncertain conditions
Garthwaite, Craig L.. 2012. The Economic Benefits of Pharmaceutical Innovations: The Case of Cox-2 Inhibitors. American Economic Journal: Applied Economics, 4(3): 116-137.